New Brunswick, N.J. (April 20, 2021) – Johnson & Johnson (NYSE: JNJ) today announced results for first-quarter 2021. “Johnson & Johnson delivered a strong first quarter performance led by the above market growth of our Pharmaceutical business and continued recovery in Medical Devices,” said Alex Gorsky, Chairman and Chief Executive Officer. “The ability to deliver these results while simultaneously advancing our robust pipeline of life-enhancing medicines, products and solutions during these times is a testament to the strength and resilience of our business and the dedication of the 135,000 employees of Johnson & Johnson who strive every day to profoundly change the trajectory of health for humanity and make healthier communities for everyone, everywhere.”
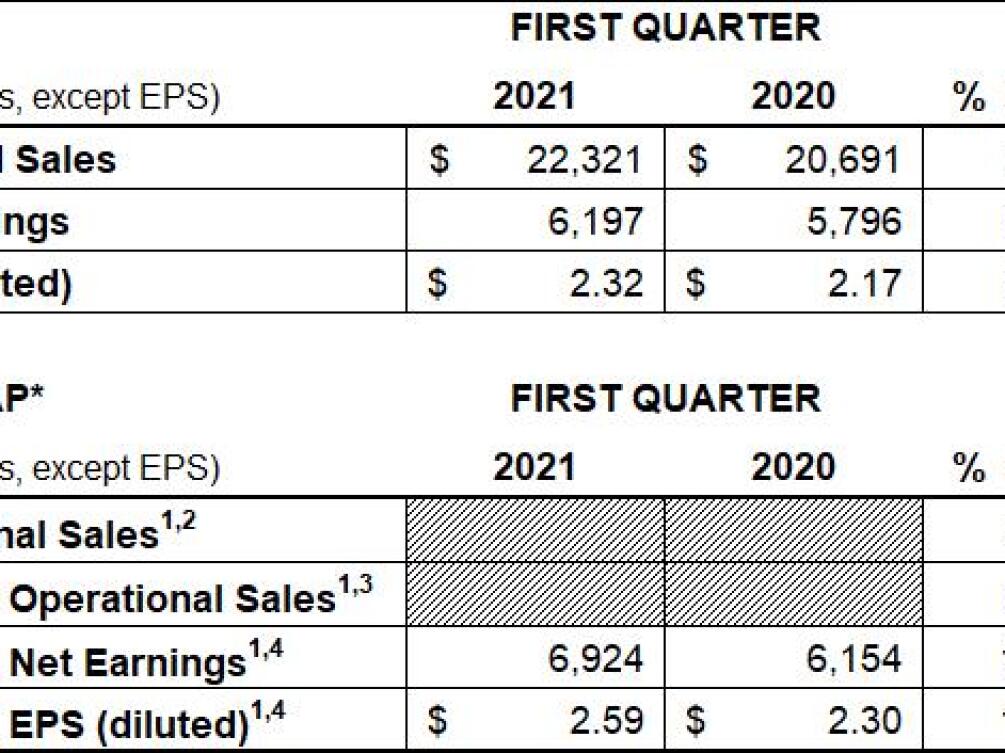
OVERALL FINANCIAL RESULTS:
1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
4 Excludes intangible amortization expense and special items
REGIONAL SALES RESULTS:
1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded
SEGMENT SALES RESULTS:
1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded
FIRST-QUARTER 2021 SEGMENT COMMENTARY:
Consumer Health
Consumer Health worldwide operational sales, excluding the net impact of acquisitions and divestitures, declined 2.9%* primarily driven by negative prior year comparisons related to the COVID-19 pantry loading in Q1 2020, mainly in over-the counter products. Partially offsetting the decline is growth in LISTERINE in oral care products, JOHNSON’S BABY in baby care products, international skin health/beauty products and NICORETTE in international over-the-counter products.
Pharmaceutical
Pharmaceutical worldwide operational sales, excluding the net impact of acquisitions and divestitures, grew 7.4%* driven by DARZALEX (daratumumab), for the treatment of multiple myeloma, STELARA (ustekinumab), a biologic for the treatment of a number of immune-mediated inflammatory diseases, ERLEADA (apalutamide), a next-generation androgen receptor inhibitor for the treatment of patients with prostate cancer, TREMFYA (guselkumab), a biologic for the treatment of adults living with moderate to severe plaque psoriasis, and for adults with active psoriatic arthritis, INVEGA SUSTENNA/XEPLION/INVEGA TRINZA/TREVICTA (paliperidone palmitate), long-acting, injectable atypical antipsychotics for the treatment of schizophrenia in adults, IMBRUVICA (ibrutinib), an oral, once-daily therapy approved for use in treating certain B-cell malignancies, a type of blood or lymph node cancer. This growth was partially offset by biosimilar and generic competition, with declines primarily in REMICADE (infliximab), a biologic approved for the treatment of a number of immune-mediated inflammatory diseases, and U.S. ZYTIGA (abiraterone acetate), an oral, once-daily medication for use in combination with prednisone for the treatment of metastatic castration-resistant prostate cancer.
Medical Devices
Medical Devices worldwide operational sales, excluding the net impact of acquisitions and divestitures, grew 8.8%*, and reflects the benefit of market recovery from COVID-19 impacts in the prior year. Contributors to growth were electrophysiology products in the Interventional Solutions business, worldwide biosurgery and energy products, and international endocutters in Advanced Surgery, wound closure products in General Surgery, contact lenses and surgery in the Vision business and trauma products in Orthopaedics; partially offset by knee products in Orthopaedics.
NOTABLE NEW ANNOUNCEMENTS IN THE QUARTER:
The information contained in this section should be read in conjunction with Johnson & Johnson’s other disclosures filed with the Securities and Exchange Commission, including its Current Reports on Form 8-K, Quarterly Reports on Form 10-Q and Annual Reports on Form 10-K. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. The reader is also encouraged to review all other news releases available online in the Investors section of the company’s website at news releases.
| Regulatory Decisions | PONVORY (ponesimod) approved by U.S. FDA, an oral treatment for adults with relapsing multiple sclerosis proven superior to (teriflunomide) in reducing annual relapses and brain lesions | (press release) |
| Johnson & Johnson Single-Shot COVID-19 Vaccine granted conditional marketing authorization by European Commission | (press release) | |
| Johnson & Johnson Single-Shot COVID-19 Vaccine granted emergency use listing by the World Health Organization | ||
| Johnson & Johnson COVID-19 Vaccine authorized by U.S. FDA for emergency use | (press release) | |
| SPRAVATO (Esketamine Nasal Spray) authorized in Europe for the rapid reduction of depressive symptoms in a psychiatric emergency for patients with major depressive disorder | (press release) | |
| MONOFOCAL INTRAOCULAR LENS - TECNIS EYHANCE AND TECNIS EYHANCE TORIC II IOLS - receives FDA approval for a next generation treatment for cataract patients | (press release) | |
| Regulatory Submission | Submission of supplemental new drug application to U.S. FDA by ViiV Healthcare for expanded use of CABENUVA (rilpivirine and cabotegravir) as an HIV treatment for use every two months | (press release) |
| Other | Janssen provides update on Phase 3 ACIS Study in patients with metastatic castration-resistant prostate cancer treated with ERLEADA (apalutamide) and ZYTIGA (abiraterone acetate) plus prednisone combination ¹ | (press release) |
| Johnson & Johnson announces advance purchase agreement with the African Vaccine Acquisition Trust for the Company’s COVID-19 vaccine candidate | (press release) | |
| PONVORY (ponesimod) receives positive CHMP opinion for the treatment of adults with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features | (press release) | |
| CAR-T Therapy Ciltacabtagene Autoleucel (Cilta-cel) accepted for accelerated assessment in Europe for the treatment of patients with heavily pretreated multiple myeloma | (press release) |
1 Subsequent to the quarter
FULL-YEAR 2021 GUIDANCE:
Johnson & Johnson does not provide GAAP financial measures on a forward-looking basis because the company is unable to predict with reasonable certainty the ultimate outcome of legal proceedings, unusual gains and losses, acquisition-related expenses and purchase accounting fair value adjustments without unreasonable effort. These items are uncertain, depend on various factors, and could be material to Johnson & Johnson’s results computed in accordance with GAAP.
| ($ in Billions, except EPS) | January 2021 | April 2021 |
Adjusted Operational Sales1,2 Change vs. Prior Year | 8.0% - 9.5% | 8.7% - 9.9% |
Operational Sales2 Change vs. Prior Year | $88.8B - $90.0B 7.5% – 9.0% | $89.3B - $90.3B 8.2% – 9.4% |
Estimated Reported Sales3 Change vs. Prior Year | $90.5B - $91.7B 9.5% – 11.0% | $90.6B - $91.6B 9.7% – 10.9% |
Adjusted Operational EPS (Diluted)2,4 Change vs. Prior Year | $9.25 - $9.45 15.2% - 17.7% | $9.30 - $9.45 15.8% - 17.7% |
Adjusted EPS (Diluted)3,4 Change vs. Prior Year | $9.40 - $9.60 17.1% - 19.6% | $9.42 - $9.57 17.3% - 19.2% |
1 Non-GAAP financial measure; excludes the net impact of acquisitions and divestitures
2 Non-GAAP financial measure; excludes the impact of translational currency
3 Calculated using Euro Average Rate: January 2021 = $1.21 and April 2021 = $1.19 (Illustrative purposes only)
4 Non-GAAP financial measure; excludes intangible amortization expense and special items
Other modeling considerations will be provided on the webcast.
WEBCAST INFORMATION:
Johnson & Johnson will conduct a conference call with investors to discuss this earnings release today at 8:30 a.m., Eastern Time. A simultaneous webcast of the call for investors and other interested parties may be accessed by visiting the Johnson & Johnson website. A replay and podcast will be available approximately two hours after the live webcast in the Investors section of the company’s website at events-and-presentations.
ABOUT JOHNSON & JOHNSON:
At Johnson & Johnson, we believe good health is the foundation of vibrant lives, thriving communities and forward progress. That’s why for more than 130 years, we have aimed to keep people well at every age and every stage of life. Today, as the world’s largest and most broadly-based health care company, we are committed to using our reach and size for good. We strive to improve access and affordability, create healthier communities, and put a healthy mind, body and environment within reach of everyone, everywhere. We are blending our heart, science and ingenuity to profoundly change the trajectory of health for humanity.
NON-GAAP FINANCIAL MEASURES:
*Operational sales growth excluding the impact of translational currency, adjusted operational sales growth excluding the net impact of acquisitions and divestitures and translational currency, as well as adjusted net earnings, adjusted diluted earnings per share and adjusted operational diluted earnings per share excluding after-tax intangible amortization expense and special items, are non-GAAP financial measures and should not be considered replacements for, and should be read together with, the most comparable GAAP financial measures. Except for guidance measures, reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the accompanying financial schedules of the earnings release and the Investors section of the company’s website at quarterly-results.
Copies of the financial schedules accompanying this earnings release are available on the company’s website at quarterly-results. These schedules include supplementary sales data, a condensed consolidated statement of earnings, reconciliations of non-GAAP financial measures, and sales of key products/franchises. Additional information on Johnson & Johnson, including adjusted income before tax by segment, a pharmaceutical pipeline of selected compounds in late stage development and a copy of today’s earnings call presentation can also be found in the Investors section of the company’s website at quarterly-results.
NOTE TO INVESTORS CONCERNING FORWARD-LOOKING STATEMENTS:
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic, economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact of patent expirations; the ability of the Company to successfully execute strategic plans; the impact of business combinations and divestitures; manufacturing difficulties or delays, internally or within the supply chain; product efficacy or safety concerns resulting in product recalls or regulatory action; significant adverse litigation or government action, including related to product liability claims; changes to applicable laws and regulations, including tax laws and global health care reforms; trends toward health care cost containment; changes in behavior and spending patterns of purchasers of health care products and services; financial instability of international economies and legal systems and sovereign risk; increased scrutiny of the health care industry by government agencies. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 3, 2021 including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” in the Company’s most recently filed Quarterly Report on Form 10-Q and the Company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Any forward-looking statement made in this release speaks only as of the date of this release. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
Press Contact:
Christina Chan
(732) 524-6297
Courtney Dugan
(347) 452-1061
Investor Contacts:
Christopher DelOrefice
(732) 524-2955
Sarah Wood
(732) 524-2617