From its earliest days, Johnson & Johnson has been committed to innovation and driving medical advancements forward. That cutting-edge work might include reinventing or refining existing medical tools, systems and machinery to make them perform stronger and better. Or it could be about taking an entirely new approach to treating a disease or condition that ends up promoting faster, safer recovery—and saves lives.
Since the 1880s, Johnson & Johnson scientists and researchers have racked up a lot of achievements—many of them the first, or one of the first, of their kind. Here’s a look at nine from the company’s long list of “firsts” in the pharmaceutical and medical technology sectors that have changed the trajectory of healthcare.
Since the 1880s, Johnson & Johnson scientists and researchers have racked up a lot of achievements—many of them the first, or one of the first, of their kind. Here’s a look at nine from the company’s long list of “firsts” in the pharmaceutical and medical technology sectors that have changed the trajectory of healthcare.
 Image Courtesy of Johnson & Johnson Archives1890
Image Courtesy of Johnson & Johnson Archives1890A better way to sterilize surgical products
After four years in business manufacturing sterile surgical supplies like gauze, sutures and cotton, Johnson & Johnson pioneers industrial steam sterilization and brings it to its production line. The new process not only speeds up output, but it ensures that each item the company manufactures is uniformly germ-free.
Steam sterilization by a machine is a major advancement compared to the standard sterilization method of the era: sanitizing products by hand, which was more laborious and time-consuming, and not always as effective. Image courtesy: ©Andrew McCaul Photography1895
Image courtesy: ©Andrew McCaul Photography1895Splints that fit properly
Inventor Revra DePuy launches the world’s first orthopedics company in Indiana and begins making the first fitted splints for the treatment of fractures, sprains and other musculoskeletal injuries.
“These fitted splints could be shaped to individual limbs to better heal orthopedic injuries, and they replaced earlier makeshift splinting methods like barrel staves (thin strips of wood used to make a barrel),” says Margaret Gurowitz, Johnson & Johnson Chief Historian. In 1998, DePuy becomes part of Johnson & Johnson’s orthopedics business.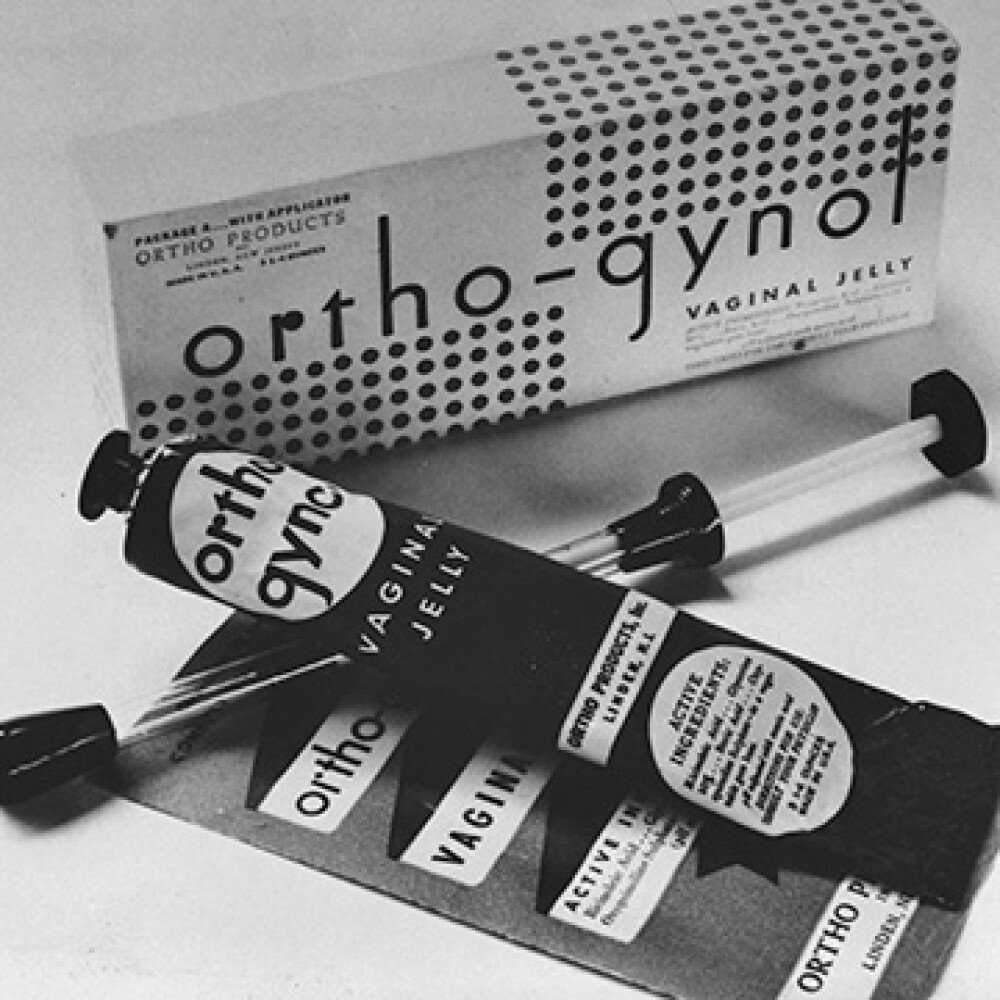 Image courtesy of Johnson & Johnson Archives1931
Image courtesy of Johnson & Johnson Archives1931Science-based birth control
Johnson & Johnson markets Ortho-Gynol, the first contraceptive gel and family planning product available by prescription. Developed at the request of doctors to fill the need for a trusted, science-based family planning product, Ortho-Gynol allows women to take charge of birth control.
Before it appeared, available contraception options included condoms, diaphragms and cervical caps. But these methods weren’t easy to obtain, since U.S. law at the time largely prohibited their sale. 1967
1967A game-changing drug for mental health
A groundbreaking prescription drug becomes the first FDA-approved antipsychotic therapy that patients could take at home—rather than under the supervision of healthcare professionals at a facility.
“Developed by Dr. Paul Janssen and first synthesized nine years earlier in 1958, this medication revolutionized the treatment of schizophrenia,” says Gurowitz. “It’s the first of many generations of medicines to treat patients with schizophrenia from the Janssen Pharmaceutical Companies of Johnson & Johnson.”
Because the drug could be taken on an outpatient basis, it relieved the burden on institutions to administer it to patients living in mental health facilities. Today, it’s still a pioneering prescription—this medication is listed on the latest World Health Organization’s List of Essential Medicines.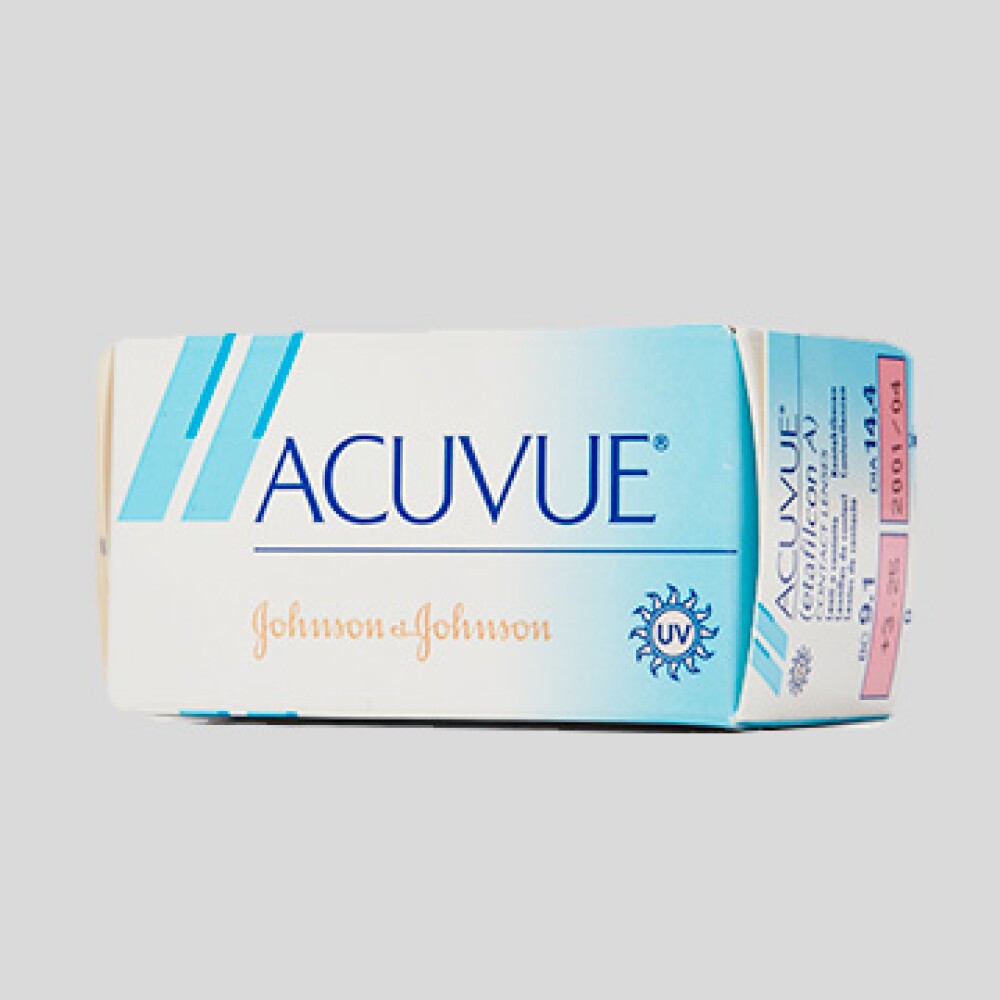 Image courtesy: ©Andrew McCaul Photography1987
Image courtesy: ©Andrew McCaul Photography1987Contact lenses meant to be discarded
Johnson & Johnson comes out with the first mass market disposable soft contact lens—and revolutionizes vision care. These contacts, available by prescription and sold under the brand name ACUVUE®, could be worn for up to a week, reducing the need for intensive cleaning and cutting back on the spread of germs.
That wasn’t the end of the company’s many eyecare innovations. In 1993, Johnson & Johnson releases the first Acuvue daily disposable contact lens; these “one and done” prescription lenses are designed to be discarded at the end of the day. Johnson & Johnson Archives1994
Johnson & Johnson Archives1994A new way to treat coronary artery disease
The company gets the green light to market the Palmaz-Schatz Balloon-Expandable Stent, one of the world’s first FDA-approved coronary stents. During a balloon angioplasty procedure to open clogged arteries, this tube-like device is inserted into a blocked artery—restoring blood flow to and from the heart and potentially saving a patient’s life.
Developed by Julio Palmaz, M.D., a vascular radiologist, and Richard Schatz, M.D., an interventional cardiologist, the stent is a game-changer in the treatment of coronary artery disease. Before coronary stents, cardiologists who performed balloon angioplasty procedures did not have a way to keep arteries open—which could lead to serious complications after the procedure. 2000
2000Medication for moderately severe Alzheimer’s Disease
Janssen gets the go-ahead to market the first medication for moderately severe Alzheimer’s disease in Europe. A year later, the medication receives approval in the United States. The medication may improve memory and cognition in people with Alzheimer’s disease and/or slow the loss of these brain functions.
A microscopic view of TB 2012A novel treatment to fight MDR-TB
Johnson & Johnson receives FDA approval for the first medicine for tuberculosis in more than 40 years with a novel mechanism of action. A year later, the World Health Organization (WHO) recommends the use of Johnson & Johnson’s medicine in combination drug therapy for people with multidrug-resistant TB (MDR-TB). Eight years later, the FDA approves this medication for children ages 5 years and older.
Tuberculosis remains one of the world’s greatest health threats, with rising rates of MDR-TB threatening the lives of millions across the globe. The development of Johnson & Johnson’s MDR-TB medicine is part of the company’s long commitment to public health and it continues to innovate to find new treatments and create access to its medicines to help end TB. 2022
2022Digital tags that make medical devices safer
In 2022, Johnson & Johnson completed a nearly decade-long effort to embed special digital tags into the barcodes on the approximately 70,000 different medical devices it sells in the United States—becoming one of the first healthcare companies in the world to do so.
These digital tags are known as UDIs, or unique device identifiers. Machine-readable UDIs allow a healthcare professional or manufacturer to obtain crucial details about the device with a simple scan of the barcode, improving patient safety (for example, by making product recalls easier) and helping healthcare providers make more informed treatment decisions.

